The welding of aluminium and its alloys
Structure of metals
Before discussing the principles by which metals achieve their mechanical strength it is necessary to have an appreciation of their structure and how these structures can be manipulated to our benefit. The simple model of an atom is of a number of electrons in different orbits circling a central nucleus. In a metal the electrons in the outer orbit are free to move throughout the bulk of the material. The atoms, stripped of their outer electrons, become positively charged ions immersed in a ‘cloud’ of negatively charged electrons. It is the magnetic attraction between the positively charged ions and the cloud of mobile, negatively charged electrons that binds the metal together. These atomic scale events give metals their high thermal and
|
|
|
|
|
|
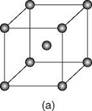
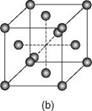
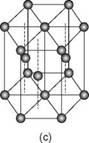
2.2 The three crystalline forms of metals: (a) body-centred cubic; (b) face-centred cubic; (c) close-packed hexagonal. (From John Lancaster, Metallurgy of Welding, 6th edn, 1999.)
electrical conductivity and the ability to deform extensively before fracturing by a process known as slip, where one plane of atoms slides over its neighbours.
In metals the atoms are arranged in a regular three-dimensional pattern repeated over a long distance on what is termed a space lattice. Conventionally, these atoms are visualised as solid spheres. The smallest atomic arrangement is the unit cell, the least complicated unit cell being the simple cube with an atom at each corner of the cube. In metals the three most common arrangements are body-centred cubic (BCC), face-centred cubic (FCC) and hexagonal close packed (HCP). Schematic views of the three structures are given in Fig. 2.2.
Each crystal structure confers certain physical properties on the metal. The face-centred cubic metals, of which aluminium is one, are ductile, formable and have high toughness at low temperatures. Although single crystals can be obtained it is more common for metals to be polycrystalline, that is, made up of a very large number of small grains. Each grain is a crystal with a regular array of atoms but at the boundaries between the grains there is a mismatch, a loss of order, in the orientation of these arrays. Both the grain boundaries and the size of the grains can have a marked effect on the properties of the metal.
2.2.1 Grain size control
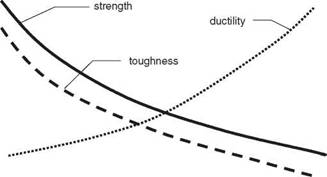
Grain size is not generally used to control strength in the aluminium alloys, although it is used extensively in reducing the risk of hot cracking and in controlling both strength and notch toughness in C/Mn and low-alloy steels. In general terms, as grain size increases, the yield and ultimate tensile strengths of a metal are reduced. The yield strength oy, is related to the grain size by the Hall-Petch equation:
s y = s і + ky d ~1/2
Mechanical
|
|
|
Increasing grain size |
|
*• |
properties
1.3 General relationship of grain size with strength, ductility and toughness.
where d is the average grain diameter, and Oi and ky are constants for the metal. Typical results of this relationship are illustrated in Fig. 2.3.
The practical consequence of this is that a loss of strength is often encountered in the HAZ of weldments due to grain growth during welding. A loss of strength may also be found in the weld metal which is an as-cast structure with a grain size larger than that of the parent metal. In the aluminium alloys the strength loss due to grain growth is a marginal effect, with other effects predominating. Grain size does, however, have a marked effect on the risk of hot cracking, a small grain size being more resistant than a large grain size. Titanium, zirconium and scandium may be used to promote a fine grain size, these elements forming finely dispersed solid particles in the weld metal. These particles act as nuclei on which the grains form as solidification proceeds.