Fuel Footprint
For a good understanding of the gasification of any particular feedstock, whether coal, heavy oil, waste biomass, or gas, it is important to calculate the gasification characteristics of the fuel in order to be able to understand what is going on in the reactor and to optimize the reaction conditions.
|
|
|
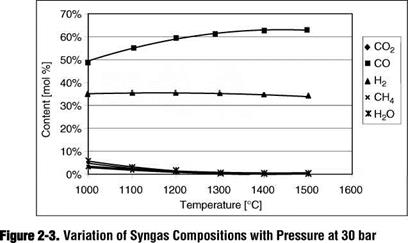
Temperature [°С] Figure 2-4. Variation of Yields with Pressure at 30 bar |
Instead of discussing this topic in the general terms of fuel, oxygen-containing gas, and moderator, we will do so in terms of carbon, oxygen, and steam. But remember, it holds for any fuel!
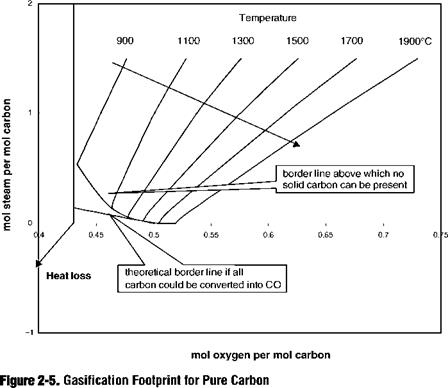
A most instructive representation of the fuel gasification characteristic is the “footprint,” which comprises a graph where for a specified heat loss from the gasification reactor and a specific set of reactants—coal, oxygen, and steam, each with a fixed temperature and composition—the steam consumption per kmole product gas is plotted along the ordinate, and the oxygen consumption per kmole of product gas is plotted along the abscissa. Such a graph made for various heat losses
forms the fuel footprint. In Figure 2-5 an example is given in which for a fixed heat loss various isotherms are drawn together with the borderline below which no complete conversion of the carbon is thermodynamically possible.
In the lower left-hand corner of Figure 2-5 the straight line represents the minimum amount of gasifying agents (oxygen and steam) that are required to gasify the coal, assuming that no H20, C02, and CH4 are present in the gas. Thermodynamically this is not possible; the real line assuming thermodynamic equilibrium, and the addition of just sufficient oxygen and steam so as to gasify all carbon, is represented by the curved line. The lower temperature part of this line is typical for fluid-bed gasifiers and, for example, the hot lower part of a dry ash moving-bed gasifier. The departure from the straight line implies that part of the steam remains unconverted for thermodynamic reasons. It also means that the CO content becomes higher. It is observed that at temperatures of about 1500°C, which are typical for dry coal feed entrained-flow slagging gasifiers, the straight line and the thermodynamic equilibrium line almost touch each other.
To generate the isotherms, the steam-to-product gas ratio is fixed above the minimum required for complete gasification at the given temperature. It is seen that in order to carry the additional steam ballast to the same high temperatures prevailing in the gasifier, more oxygen is required. This is hence a less efficient operation.
|
|
The surplus of gasifying agent implies that no carbon can be present. Therefore the two equilibrium constants of the CO shift and the steam methane reforming equation will have to be used in the calculations instead of those of the three heterogeneous equations (Boudouard, water gas, and hydrogasification). Because the steam-to-product gas ratio is known, the number of equations and variables remain identical and the algorithm can be solved.
In practice, one will never want to operate at the thermodynamic equilibrium line, as carbon formation may occur at the slightest operational disturbance. Typically, for dry coal feed slagging gasifiers it is assumed that less than 1 % of the carbon remains unconverted, hidden in ash particles, and l-1.5mol% of C02 is present in the gas. This gives some room for process control.