Devolatilization
The first step, heating up of the coal particles, is in one sense the simplest part of the process. Nonetheless, the speed at which it takes place has an influence on the subsequent steps, so that it is of great importance in any accurate model.
Devolatilization takes place already at low temperatures (350-800°C) and in parallel with the heating up of the coal particles. The rate of heating of the coal particles influences the way in which the devolatilization takes place (Jiintgen and van Heek 1981, p. 65). The rate of devolatilization is dependant not only on the rate of heating, however, but also on the particle size and the rate of gasification by the water gas reaction, and hence on the reaction temperature and the partial pressure of steam.
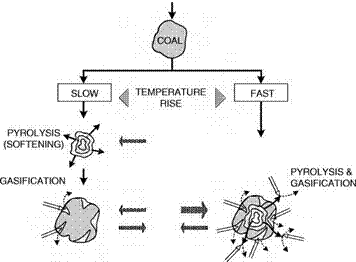
The interplay between pyrolysis and gasification under different heating conditions is shown in Figure 3-2. If the heating up is slow then the pyrolysis reactions set in from about 350°C. The gasification reaction of both volatiles and char with steam is very slow at this temperature. The concentration of volatiles outside the particle increases rapidly, and gasification only sets in after devolatilization is complete. If, however, the rate of heating is high, then both pyrolysis and gasification take place simultaneously, so that a high concentration of volatiles is never allowed to build up. This is one reason why high-temperature entrained-flow reactors produce a clean gas in such a short time. Compare this with a counter-current moving-bed process where lump coal is used. The heating up rate is slow and a high volatiles concentration is built up and removed unreacted from the reactor by the syngas.
For finely pulverized coal particles at high temperature the residence time is very short (10-200 ms) (Smoot and Smith 1985, p. 55 ff). The extent of devolatilization is highly dependant on the final temperature and can vary considerably from that found by performing a proximate analysis in accordance with ASTM (American Society for Testing and Materials), DIN (Deutsches Institut fur Normung), or other standard methods. The product distribution of the devolatilization process also varies significantly with changes in the pyrolysis temperature and the speed of heating up.
Although devolatilization processes during gasification and combustion are thought to be generally similar, the fact that many gasification processes operate at
|
|
 |
HEAT FLOW
Figure 3-2. Influence of Heating Rate on Gasification Process (Source: Jiintgen and van Heek 1981)
higher pressures has to be taken into account. The weight loss due to devolatilization can be of the order of 10% less at typical gasifier pressures of 30 bar.