DEDUCTIONS FROM THE THERMODYNAMIC MODEL
1.1.1 Effect of Pressure
There is considerable advantage to gasifying under pressure, sufficiently so that practically all modern processes are operated at pressures of at least 10 bar and up to as high as 100 bar. The reasons for this are savings in compression energy and reduction of equipment size. To appreciate the realities of savings in compression energy, we can compare the energy required to provide 100,000 Nm3/h raw synthesis gas at 45 bar by either
1. gasifying at a relatively low pressure (5 bar) and compressing the synthesis gas, or alternatively,
2. compressing the feedstocks to 55 bar (allowing for pressure drop in the system) and gasifying at the higher pressure.
For the calculations we will use an oil feedstock, so as to include simply the energy for raising the fuel pressure in case 2. It is also assumed that oxygen is available from an air separation unit at atmospheric pressure in both cases. The energy is given as shaft energy on the machines. The enthalpy difference of the moderating steam in the two cases is neglected (see Table 2-1).
|
Table 2-1 Comparison of Compression Energy for Low and High Pressure Gasification |
|||
|
5 Bar |
50 Bar |
||
|
Gasification |
Gasification |
||
|
Feed pumping energy |
35,450 kg/h |
0.03 MW |
0.09 MW |
|
Oxygen compression |
21,120Nm3/h |
2.85 MW |
4.97 MW |
|
Syngas compression |
100,000 Nm3/h |
19.70 MW |
0.00 MW |
|
Total |
22.58 MW |
5.05 MW |
The pressure in a gasifier is therefore generally selected in accordance with the requirements of the process or equipment upstream or downstream of the gasifier. For extremely high pressures, as required by ammonia synthesis (130-180 bar), for example, this argument ceases to be dominant, as gasification at pressures above 70-100 bar become impractical for equipment reasons. When the gas is to be used in a combined cycle (CC) power station where the gas turbine requires a pressure of say 20 bar, the gasifier pressure may operate at a somewhat higher pressure than this to allow for pressure losses between the gasifier and the gas turbine.
Strictly speaking, allowance should be made in preparing the above figures for the fact that the syngas (CO + H2) yield and the heating value of the gas are somewhat different at the two gasification pressures. This slight difference does not, however, alter the conclusion that, other things being equal, compression of the reactants is energetically superior to compression of the gasifier product gas. Other considerations when selecting a gasification pressure are discussed in Section 6.1.
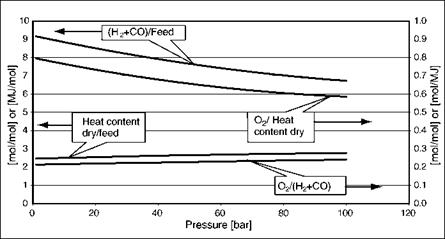
It is instructive to see how the gas composition changes with pressure, and this is shown in Figures 2-1 and 2-2 with the calculations all performed at 1000°C. The increase in methane and C02 content in the synthesis gas with increasing pressure can be seen clearly in Figure 2-1. In Figure 2-2 it is plain that the yield of synthesis gas (as H2 + CO) drops with pressure, whereas the heat content yield increases (reflecting the higher methane content). Similarly, the variation in oxygen demand goes in opposite directions depending on whether it is expressed per unit of syngas or per unit of heating value.
We will return to these effects later in the chapter, when looking at the differences between optimizing for IGCC and for synthesis gas applications.
If we repeat the above calculations at, say, 1500°C, we see in principle the same trends with increasing pressure. However, if we look at the actual numbers in Table 2-2, then we notice that at this temperature the actual changes of gas composition with pressure are almost negligible.
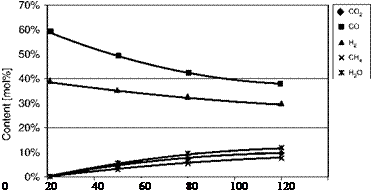 |
Figure 2-1. Variation of Syngas Compositions with Temperature at 1000°C
|
Figure 2-2. Variations of Yields with Temperature at 1000°C 2.3.2 Effect of Temperature |
The temperature is generally selected on the basis of the ash properties (i. e., below the softening point of the ash for fluid-bed and dry ash moving-bed gasifiers and above the melting point for slagging gasifiers). For coals with very high ash melting points it is often advantageous to add flux to the coal feed in order to lower the ash melting point. As will be discussed later, gasifying at very high temperatures
|
Table 2-2 Variations of Syngas Compositions and Yields at 1500°C |
||||
|
1 Bar |
30 Bar |
60 Bar |
100 Bar |
|
|
C02, mol% |
0.00 |
0.01 |
0.21 |
0.34 |
|
CO, mol% |
63.42 |
63.33 |
62.88 |
62.88 |
|
H2, mol% |
34.37 |
33.89 |
33.07 |
33.07 |
|
CH4, mol% |
0.01 |
0.27 |
0.85 |
0.85 |
|
H20, mol% |
0.01 |
0.21 |
0.67 |
0.67 |
|
Others, mol% |
2.19 |
2.20 |
2.20 |
2.19 |
|
Total, mol% |
100.00 |
100.00 |
100.00 |
100.00 |
|
H2+CO/feed, mol/mol |
8.61 |
8.52 |
8.45 |
8.36 |
|
LHV dry/feed, M J/mol |
2.31 |
2.31 |
2.32 |
2.32 |
|
02/(H2+C0), mol/mol |
0.27 |
0.27 |
0.27 |
0.27 |
|
02/LHV dry, mol/MJ |
0.99 |
0.99 |
0.98 |
0.97 |
will increase the oxygen consumption of a gasification process and will reduce the overall process efficiency.
For process control purposes where ratios between fuel, oxygen, and/or steam are known, the temperature can be calculated. This is an important aspect, as temperatures in slagging gasifiers can only be measured with great difficulty and are generally not very trustworthy.
Since most modern gasification processes operate at pressures of 30 bar or higher, temperatures of above 1300°C are required in order to produce a synthesis gas with a low methane content. The fact that such a high temperature is required in any case for thermodynamic reasons is why there is little scope for the use of catalysts in gasifiers. The use of catalysts is restricted to clean gasification environments that are only encountered in the partial oxidation of natural gas and in steam methane or naphtha reforming.
This observation leads us on to perform the same exercise of investigating the variations of gas compositions and yield with temperature as shown in Figures 2-3 and 2-4.
In Figures 2-3 and 2-4 we can see that with increasing temperature the gas becomes increasingly CO rich. In Figure 2-4 the increased oxygen demand at high temperature is apparent. The H2+CO yield goes through a mild maximum between 1200°C and 1300°C.