REACTIONS
During the process of gasification of solid carbon whether in the form of coal, coke, or char, the principle chemical reactions are those involving carbon, carbon monoxide, carbon dioxide, hydrogen, water (or steam), and methane.
These are:
Combustion reactions,
C + Vi02=C0 -111 MJ/kmol (2-1)
CO + Vi 02 = C02 -283 MJ/kmol (2-2)
H2 + Vi 02=H20 -242 MJ/kmol (2-3)
the Boudouard reaction,
C + C02^2 CO +172 MJ/kmol (2-4)
the water gas reaction,
C + H2O^CO + H2 +131 MJ/kmol (2-5)
and the methanation reaction,
C +2 H2 S CH4 -75 MJ/kmol (2-6)
As reactions with free oxygen are all essentially complete under gasification conditions, reactions 2-1, 2-2, and 2-3 do not need to be considered in determining an equilibrium syngas composition. The three heterogeneous (i. e., gas and solid phase) reactions 2-4, 2-5, and 2-6 are sufficient.
In general, we are concerned with situations where also the carbon conversion is essentially complete. Under these circumstances we can reduce equations 2-4, 2-5, and 2-6 to the following two homogeneous gas reactions:
CO shift reaction:
and the steam methane reforming reaction:
![]() CH4 + H20 S C02 + 3 H2 + 206 MJ/kmol
CH4 + H20 S C02 + 3 H2 + 206 MJ/kmol
Note that by subtracting the moles and heat effects from reaction 2-4 from those in reaction 2-5 one obtains reaction 2-7, and by subtracting reaction 2-6 from 2-5 one obtains reaction 2-8. Thus reactions 2-7 and 2-8 are implicit in reactions 2-4, 2-5, and 2-6. But not the other way around! Three independent equations always contain more information than two.
Reactions 2-1, 2-4, 2-5, and 2-6 describe the four ways in which a carbonaceous or hydrocarbon fuel can be gasified. Reaction 2-4 plays a role in the production of pure CO when gasifying pure carbon with an oxygen/C02 mixture. Reaction 2-5 takes a predominant role in the water gas process. Reaction 2-6 is the basis of all hydrogenating gasification processes. But most gasification processes rely on a balance between reactions 2-1 (partial oxidation) and 2-5 (water gas reaction).
For real fuels (including coal, which also contains hydrogen) the overall reaction can be written as
![]()
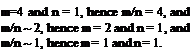 |
|
CnHm + n/2 02 = n CO + m/2 H2
Gasification temperatures are in all cases so high that, thermodynamically as well as in practice, apart from methane, no hydrocarbons can be present in any appreciable quantity.