The welding of aluminium and its alloys
Hot cracking
Hot cracking is a welding problem that does not occur in pure metals but may be found in certain alloy systems. It is not confined to the aluminium alloys but is also encountered in steels, nickel and copper alloys. The funda
mental mechanism is the same in all of the alloy systems and is a function of how metal alloy systems solidify. As the name suggests, this is a high- temperature cracking mechanism which, because of its prevalence, is known by a number of different names - hot cracking, hot fissuring, hot shortness, liquation cracking, centre-line cracking or solidification cracking.
The addition of alloying elements to a pure metal will cause a change in the freezing temperature of the alloy from that of the pure metal and may result in a number of different phases - a solid solution, a eutectic and an intermetallic compound, for instance, being produced. These changes of state and the relative proportions of each phase are represented on phase diagrams. It is not intended to go into any greater detail than this - for further information refer to the books listed in the Bibliography. The lowest melting point composition of the alloy is known as the eutectic composition which freezes at one specific temperature. The other non-eutectic compositions freeze over a range.
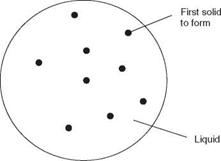
It is necessary next to look at how a metal solidifies. Figure 2.11 shows the way in which the lowest melting point constituents are pushed to the grain boundaries by the solidification fronts as the solid particles grow in size.
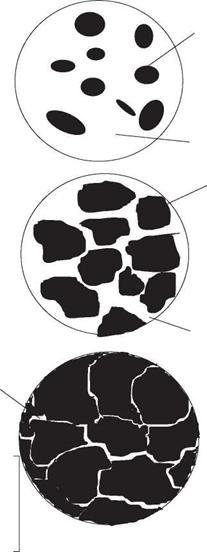
The first solid to form is a unit cell that acts as a nucleus to which atoms attach themselves, forming what is known as a dendrite. The dendrite increases in size until such time as it begins to collide with its neighbours that have been nucleating and growing in a similar manner. The point at which this collision takes place becomes the boundary between adjacent dendrites, crystals or grains - the grain boundary. Since almost all alloy systems, except eutectics, solidify over a range of temperatures, it is common sense to expect that the first metal to solidify will be the highest melting/freezing point alloy and the last to be the lowest melting point composition, always the eutectic if one has formed. The consequence of this solidification process is that the lowest melting point alloy composition is pushed ahead of the solidifying dendrite until it becomes trapped between the adjacent dendrites, i. e. along the grain boundaries. If the difference in melting point between the low melting point eutectic and the bulk of the metal is sufficiently great then the liquid film along the grain boundaries may part as the metal cools and contracts. The results of this are illustrated in Fig. 2.12.
In most metals this effect is caused by tramp elements or impurities. Sulphur in steel and nickel alloys is a good example where low melting point sulphide eutectics are formed. In the aluminium alloys, however, it is the deliberately added alloying elements themselves that form a range of eutectics with freezing points substantially lower than the bulk metal. This means that all aluminium alloys are susceptible to some degree to this form of cracking, differing only in their degree of susceptibility. Cracking tests have
|
|
|
Small amount of lowest melting point liquid along the grain boundaries |
|
Increasing area of solid |
|
Liquid |
|
Increasing amounts of solid phase |
|
Small amounts of liquid surrounding large islands of solid |
|
|
|
Note that a large difference in melting point between the bulk of the metal and the ‘low’ melting point films results in an increased sensitivity to hot or solidification cracking |
1.11 Solidification of a metal.
|
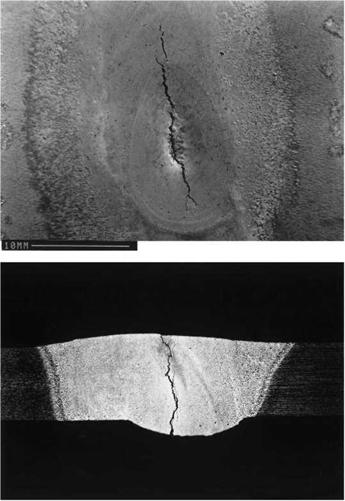
2.12 Solidification cracking: (a) in the finish crater of a TIG weld in A5083 alloy; (b) in a 3 mm thick A6082 plate/4043 filler metal TIG weld. Courtesy of TWI Ltd. |
determined what is termed the hot short range, the range of composition within which the alloy has a high risk of hot cracking. The hot short range of the common alloying elements is given in Table 2.4.
These results are produced by performing standard cracking tests. These tests are designed to load the weld transversely under controlled conditions to give cracks, the length of which will be a measure of the crack sensitivity of the specific alloy being tested. This enables the alloys to be ranked in order of sensitivity and characteristics such as the hot short range to be determined.
28 The welding of aluminium and its alloys Table 2.4 Hot short range and eutectic characteristics
|
Alloy system Hot short range Eutectic composition % -------------------------------------------------------------- Temp. (°C) Composition (%)
|
|
|
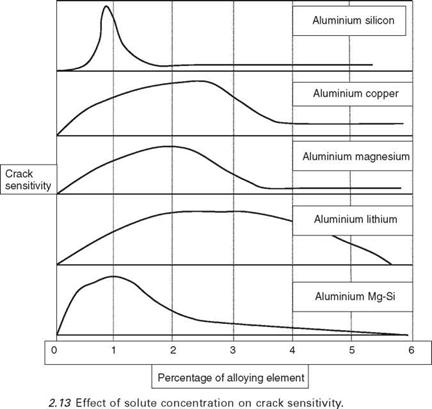
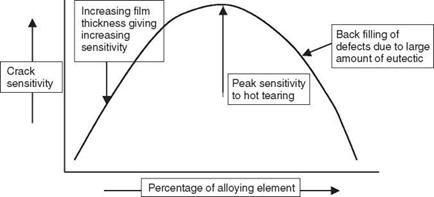
The aluminium alloys all exhibit a peak in sensitivity with a high resistance to hot cracking at both low and high alloy content, as shown in Fig. 2.13. At low levels of alloy content there is only a small amount of eutectic present. This results in the liquid film on the grain boundaries being
|
2.14 Generalised picture of crack sensitivity. |
either discontinuous or very thin. The strength of a liquid film can be derived from
F = kgA t
where F = force required to tear the liquid;
k = a constant;
g= the liquid/solid interfacial tension;
A = cross-sectional area;
t = liquid film thickness.
Therefore, as the liquid film thickness t increases, the force required to tear the film F reduces. The force required for cracking begins to increase, however, once there is sufficient eutectic available that it can begin to flow into and fill any cracks that form. The crack sensitivity therefore drops, the cracks heal themselves and a crack-free structure results. This is a very useful feature when welding alloys that are sensitive to liquation cracking in the HAZ. Figure 2.14 illustrates this graphically, where it can be seen that the shape of the curve is essentially the same as that in Fig. 2.13.
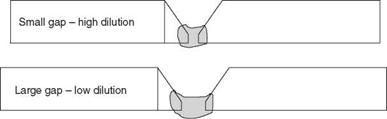
The practical consequence of this is that the crack susceptibility of the weld metal is very sensitive to changes in composition. In very many situations when welding aluminium alloys, the filler metal does not match the parent material. It is most important that this fact is realised and that account is taken of the composition of the resultant weld metal. There are a number of other factors, apart from filler metal and parent metal composition, which affect the weld metal composition. Fit-up of the component parts can affect the amount of dilution in a joint, dilution being the amount of parent metal dissolved into the weld metal during welding. In the root pass a wide gap will give low dilution, a narrow gap high dilution, as illustrated in Fig. 2.15.
|
2.15 Effect of variations in root gap. |
|
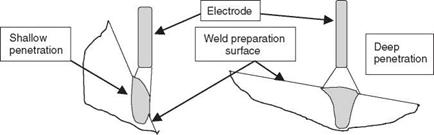
2.16 Effect of electrode angle on dilution. |
A steep weld preparation bevel angle will give lower dilution than a wide shallow weld preparation because of the change in the angle of the electrode to the weld bevel, as shown in Fig. 2.16. Changing the welding process or the welding parameters, particularly the welding current, may also affect penetration and therefore dilution. From a shop-floor point of view this means that weld bevel angles, joint fit up and welding parameters need to be controlled far more closely than in the case of steel welding if problems of hot cracking are to be avoided.
In summary, if hot cracking is encountered it may be eliminated by one or more of the following:
• A small grain size. It has been found that small additions of elements such as titanium, zirconium or scandium will act as nuclei for the formation of a very fine grain during solidification. Filler metals can be purchased that are alloyed with titanium and/or zirconium.
• Control the composition of the weld pool by adding filler metal to produce an alloy that is not in the hot short range.
• Use an edge preparation and joint spacing to permit sufficient filler metal to be added to achieve a weld metal composition outside the hot short range.
• Use the highest welding speed. High speeds reduce the length of time the weld is within the hot short temperature range. High welding speeds also reduce the size of the HAZ and consequently the shrinkage stresses across the joint.
• Use high-speed, small-volume multi-run procedures instead of large volume, single run deposits.
• Select welding and assembly sequences that minimise restraint and residual stresses.
• Apply an external force to maintain the weld in compression while it is in the hot short range.
• Select a filler metal with a melting point close to that of the parent metal, see Appendixes C and D.