The welding of aluminium and its alloys
Precipitation (age) hardening
Microstructures with two or more phases present possess a number of ways in which the phases can form. The geometry of the phases depends on their relative amounts, whether the minor phase is dispersed within the grains or is present on the grain boundaries and the size and shape of the phases. The phases form by a process known as precipitation, which is both time and temperature controlled and which requires a reduction in solid solubility as the temperature falls, i. e. more of the solute can dissolve in the solvent at a high temperature than at a low temperature. A simple analogy here is salt in water - more salt can be dissolved in hot water than in cold. As the temperature is allowed to fall, the solution becomes saturated and crystals of salt begin to precipitate.
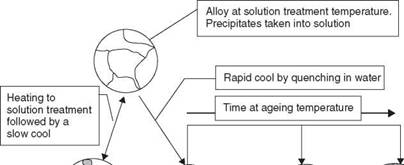
A similar effect in metals enables the microstructure of a precipitation hardenable alloy to be precisely controlled to give the desired mechanical properties. To precipitation or age harden an alloy the metal is first of all heated to a sufficiently high temperature that the second phase goes into solution. The metal is then ‘rapidly’ cooled, perhaps by quenching into water or cooling in still air - the required cooling rate depends upon the alloy system. Most aluminium alloys are quenched in water to give a very fast cooling rate. This cooling rate must be sufficiently fast that the second phase does not have time to precipitate. The second phase is retained in solution at room temperature as a super-saturated solid solution which is metastable,
|
|
SHAPE * MERGEFORMAT

Annealed structure - coarse
precipitates on the grain boundaries
Solution treated - precipitates retained in solution
Correctly aged - fine dispersion of precipitates within the grains
Overaged - coarse precipitates within the grains
2.6 Illustration of the solution treatment and age-(precipitation) hardening heat treatment cycle.
that is, the second phase will precipitate, given the correct stimulus. This stimulus is ageing, heating the alloy to a low temperature. This allows diffusion of atoms to occur and an extremely fine precipitate begins to form, so fine that it is not resolvable by normal metallographic techniques. This precipitate is said to be coherent, the lattice is still continuous but distorted and this confers on the alloy extremely high tensile strength. In this world, there is no such thing as a free lunch, so there is a marked drop in ductility to accompany this increase in strength.
If heating is continued or the ageing takes place at too high a temperature the alloy begins to overage, the precipitate coarsens, perhaps to a point where it becomes metallographically visible. Tensile strength drops but ductility increases. If the overageing process is allowed to continue then the alloy will reach a point where its mechanical properties match those of the annealed structure.
Too slow a cooling rate will fail to retain the precipitate in solution. It will form on the grain boundaries as coarse particles that will have a very limited effect on mechanical properties. The structure is that of an annealed metal with identical mechanical properties. The heat treatment cycle and its effects on structure are illustrated in Fig. 2.6.
|
Table 2.1 Summary of mechanical properties for some aluminium alloys
|
|
UTS: ultimate tensile strength |
Summary
This chapter is only the briefest of introductions to the science of metals, how crystal structures affect the properties and how the fundamental mechanisms of alloying, hardening and heat treatment, etc., are common to all metals. Table 2.1 gives the effects of solid solution strengthening, cold working and age hardening. It illustrates how by adding an alloying element such as magnesium, the strength can be improved by solid solution alloying from a proof strength of 28N/mm2 in an almost pure alloy, 1060, to 115 N/mm2 in an alloy with 4.5% magnesium, the 5083 alloy. Similarly, the effects of work hardening and age hardening can be seen in the increases in strength in the alloys listed when their condition is altered from the annealed (O) condition. Note, however, the effect that this increase in strength has on the ductility of the alloys.