New developments in advanced welding
Materials used in tubular cored wire welding
Early flux-cored wires were developed by covered electrode developers who simply took formulations from MMA consumables and turned the product inside out. However, they failed to capitalise on the great potential advantage of the tubular form: while MMA electrodes need a binder to stick the powder to the core wire, in tubular wires the powder can usually be held within the sheath without the use of binders. Since the binders, alkali metal silicates, are the main source of moisture pickup in MMA electrodes, it might have been expected that their removal from flux-cored wire would be a first priority, but instead they lingered on for many years as arc stabilisers. Flux-cored wires gained a reputation for high hydrogen contents and porous welds which they did not live down until the 1980s.
Basic MMA electrodes were based on a simple lime-fluorspar-silica (CaCO3- CaF2-SiO2) flux system, which translated easily to tubular wires. It was only necessary to lower the lime-to-fluorspar ratio to prevent excessive evolution of CO2 as the wire was heated in the electrode extension, which would cause the wire seam to blow open towards the tip. Basic slag systems produce weld metals low in oxygen, which is good for ductility and toughness. Unfortunately, oxidation of the surface of the transferring droplet is the best way to lower its surface tension so that fine droplets become stable: since this does not happen with basic wires, the metal transfer tends to be quite globular. The use of electrode negative polarity is recommended for many basic wires as this can give a finer droplet transfer at low currents. As with basic MMA electrodes, the fluorspar inhibits hydrogen pickup by the droplets, so early basic flux-cored wires gave weld metals much lower in hydrogen than those from rutile wires. In addition, given that the metal transfer would never be very smooth, developers tended to forego the use of alkali metal-based arc stabilisers, so producing relatively non-hygroscopic wires.
The latest rutile wires come very close to matching basic wires in many aspects of their performance, including weld metal hydrogen levels, but in one aspect they are left behind. The low oxygen basic weld metals have the potential to give good toughness at much higher strength levels than rutile types do. As the use of steels with yield strengths exceeding 700 MPa increases, and especially as the use of strain-based design requires the weld metal strength to overmatch that of the parent material, basic wires may be the only way to achieve satisfactory mechanical properties. This will certainly be a challenge to consumable developers, since lime-fluorspar slags are inherently fluid and difficult to manage in positional welding, while the globular transfer leads to some spatter. Help may be at hand in the form of advanced power sources: it has even proved possible in the laboratory to weld X100 pipes in the fixed position with a basic wire giving a yield strength of more than 800MPa, using pulsed arc welding.
Other areas where basic wires have traditionally been used rely on the slag fluidity to allow gases to escape and minimise porosity. Thus when welding over surfaces contaminated with oil or grease, or with thick primer coatings, basic wires may still be the best choice.
Rutile flux-cored wires have suffered by association with rutile stick electrodes, which are perceived as easy to use but high in hydrogen and unable to deliver a high level of mechanical properties. However, designers of gas-shielded tubular wires have a freer hand - unlike rutile MMA electrode designers, for example, they do not have to rely on steam as a shielding medium. Over the years, a great variety of rutile flux-cored wire formulations has been tried, but the defining characteristic has been the ability of the wire to give extremely smooth, free flight metal transfer over a wide range of currents.
As discussed above, the factor which above all controls the size of the transferring droplets is their oxygen level. It is quite possible to formulate a rutile wire which will give a low oxygen weld metal, but it then reverts to the globular transfer characteristic of basic types. In moving from low strength rutile wires to wires giving up to 700 MPa yield strength, designers might lower the weld oxygen level from 650 to 550ppm, but any further decrease would be likely to lead to unacceptable welding characteristics. At this strength level, the toughness obtained with rutile wires cannot therefore match that from basic wires, whose weld metals would have oxygen levels of 450ppm or less. Nevertheless, a considerable metallurgical feat was achieved by a number of consumable manufacturers in the 1980s when they produced rutile wires that reached the levels of charpy and crack tip opening displacement (CTOD) toughness demanded for offshore platforms for the North Sea. The use of microalloying with titanium and boron, although not always apparent in published product specifications, played a large part in this.
Another characteristic contributing to the versatility of rutile slag systems is their ability to develop a range of melting points and viscosities. Rutile melts between 1700 and 1800 °C, so with the addition of suitable fluxing agents it is easy to make slags with melting points around 1200 °C and to fine tune these according to the application. Thus, for uphill welding where the slag has to support the weld metal and mould it to a flat contour, rutile wires are pre-eminent. With some wires, currents up to 300 A can be used for vertical-up welding without losing control of the pool.
For high current downhand fillet welding, on the other hand, a slower freezing but more viscous slag may give the best results and this too can be readily formulated on a rutile base. Many such wires were in the past designed to run with CO2 shielding, since this allows cooler running of the torch and is more comfortable for the operator, but where mechanisation or automation is possible, there is a tendency to replace them with metal-cored wires running on gas mixtures rich in argon.
A survey of rutile flux-cored wires on the British market in 19685 found weld metal hydrogen contents up to 31ml/100g. Those giving the highest values contained significant amounts of hygroscopic synthetic titanates and were produced by a drawing process using solid soap which was left on the wire surface. Already, other wires in the survey, using better formulations and a soap-free production route, were giving less then 10ml/100g of deposited metal hydrogen and pointing the way to today’s figures of less than 5ml/100g for many wires. Rutile flux-cored wires are easy to use and are available for many types of steel, from mild steel to high strength and creep - resisting steels. It is therefore not surprising that they have overtaken basic types in popularity in the last 20 years, and growth in their use is expected to continue.
Metal-cored wires were patented in 1957,6 mainly as a means of overcoming a current shortage of solid welding wire. A later patent of 19743 described the addition of small amounts of non-metallic material to the core together with the metal powders, and mentions some of the features that have subsequently made metal-cored wires so useful in their own right.
While a high deposition rate per ampere may not in itself be a deciding factor in wire selection, metal-cored wires add to this a good underbead profile on argon-rich gases, which reduces defect incidence. This makes them particularly suited to fillet welding, as in shipyards and in the manufacture of earth-moving equipment. Slag levels are low, so it is possible to make three or more runs without deslagging. The lack of slag also makes welding over primers easier.
Like solid wires, metal-cored wires must be used in the dip transfer mode when welding uphill, which means that deposition rates are limited and they may not offer much advantage over solid wire. However, where vertical - down welding is permitted, very high deposition rates can be achieved: for example, a 1.2 mm wire running at 280A can deposit 5.5 kg/h. Another approach to positional welding is the use of a pulsed arc. This allows a deposition rate of about 2.3 kg/h in the uphill direction and is very suitable for robotic applications where guidance is by through-arc sensing.
Mechanisation allows metal-cored wires to be used at high productivity and the recent commercial development of tandem pulsed arc welding takes this a step further. In this system, the wires are fed through contact tips which are insulated from each other but share a gas shroud. If the pulses on the wires are 180° out of phase, the arcs do not interfere with each other, although other methods are also possible. This system has proved very effective in shipbuilding and is now starting to be used for the circumferential welding of pipelines.
The introduction of metal-cored wires in Europe coincided with a period of active involvement of the gas companies in the welding field and was used by them to promote the sales of argon-rich gases. The 1974 patent,3 which mentioned that metal-cored wires may be formulated to run well under CO2 shielding, was forgotten for a number of years. However, in Japan, the welding consumables industry remained independent of gas suppliers and such wires became popular. Now that less of the European welding industry belongs to gas companies, metal-cored wires for use under CO2 are available from European manufacturers as well.
Although self-shielded tubular wires of large diameter were made in Austria before World War II,2 the pedigree of products currently on the market is generally considered to date back to the late 1950s, when new wires were announced almost simultaneously in the USA7 and the USSR.8 The driving force was the need for a product which would be faster to use than stick electrodes, but which would be independent of a supply of shielding gas. The latter is important not only in areas where the infrastructure for supply does not exist, but also, for example, in welding on tall structures where heavy gas bottles could be hazardous.
Arc welding requires the pool and transferring metal to be protected from atmospheric contamination by oxygen and nitrogen, which cause porosity and degrade the mechanical properties of the weld metal. In the absence of an external shielding gas, it was found that the lime-fluorspar system borrowed from basic stick electrodes produced a gas shield if the wire stickout was long enough for the heat generated to break down the lime into CO2 and CaO, while the fluorspar vaporised in the arc. The addition of metallic aluminium, as a deoxidant and nitride former, allowed more normal stickouts to be used and a range of wires was produced which could be used without external shielding gas.
Early self-shielded wires were not remarkable for their mechanical properties, mainly because the shielding did not altogether exclude nitrogen but trapped much of it as aluminium nitride in the weld metal, while the excess aluminium remained in solution in the weld metal. Aluminium is a strong ferrite former and if sufficient is present to reduce austenite formation, the beneficial austenite transformation products that give steel its combination of strength and toughness may not be formed. Much ingenuity went into developing other shielding mechanisms, involving for example lithium compounds which produce metallic lithium in the arc, so that aluminium levels could be reduced and the toughness improved. So successful were these efforts that by the mid-1970s, self-shielded wires were being used to weld thick section offshore platforms for the Forties Field in the North Sea, meeting stringent charpy and CTOD requirements.
A particular advantage of self-shielded wires in that application, as in the welding of high-rise buildings, was their relative immunity to winds and draughts. This is because the metal vapour shielding is not easily blown away and the weld is still rich in nitride formers and deoxidants.
Over the years, self-shielded wires were developed for many different applications, from high deposition rate welding of heavy plate to the welding of thin sheet at low currents and voltages. Special wires have been made for semi-automatic welding of pipeline girth welds, for welding galvanised steel and for ‘gasless electrogas’ welding. All these are excellent products and to try them, or to read the patents which describe them, is to be impressed by the way in which physical, chemical and metallurgical challenges have been overcome to produce them. Nevertheless, the materials which provide the shielding need significant amounts of energy to melt or vaporise them so that they can do their work, and this reduces deposition rates compared with gas - shielded wires. Moreover, the metal vapours eventually condense to form either particulate fume or condensates on any exposed surfaces around the weld. The high level of deoxidation increases the surface tension of the droplets, making it difficult to achieve fine droplet transfer. The use of rutile to control slag viscosity is ruled out in products that aim to have high toughness because in the presence of strong deoxidants, metallic titanium is reduced into the weld metal, where it leads to severe secondary hardening and embrittlement by forming titanium nitrides.
For these reasons, self-shielded wires tend not to be the consumables of choice where the option of a gas-shielded product is open. A further reason often cited is the use, in many self-shielded wires, of barium compounds. These have low work functions and reduce the voltage drop at the arc cathode, allowing barium-containing wires to operate at voltages up to 8 V less than their barium-free counterparts. This reduces nitrogen pickup and, together with the very low latent heat of fusion of barium compounds, increases the welder’s feeling of control in positional welding. Unfortunately, some barium compounds are toxic, and although those used in self-shielded wires are insoluble and there is no epidemiological evidence of harm to welders’ health, there are concerns about whether macroscopically insoluble compounds might be absorbed by the welder if present as sufficiently fine particulates in the fume.
There is certainly a place for self-shielded wires. They are convenient to use, particularly suitable for outdoor applications and torches are lightweight. Indeed new self-shielded wires are still actively being developed. However, users concerned with the highest productivity may conclude that gas-shielded consumables have more to offer.
2.4.5 Wires for stainless steels
Tubular wires for welding stainless steels may be of any of the types described above, but have some special characteristics of their own. Early wires were often made with basic slag systems, but since neither hydrogen cracking nor toughness is a problem with most stainless steels, attention soon moved to rutile types, which promise great ease of use and excellent weld appearance. The lower melting point of stainless weld metal compared with low alloy types means that for positional welding, more support from the slag is needed. This means that with stainless flux-cored wires, the difference between the all-positional types with fast freezing slags and the downhand types with more fluid ones is more marked than it is with mild steel wires and it is worthwhile to choose the right wire for the application.
Many stainless flux-cored wires are of Japanese origin and were designed for CO2 shielding. Users should be aware that while these are capable, where required, of depositing weld metals with less than 0.04% C, for lower levels an argon-rich shielding gas is likely to prove more reliable. Metal-cored stainless wires are available and are especially suited to use with pulsed power sources, provided these are suitably programmed - programs for mild steel wires will not be optimised for stainless ones. It then becomes possible to weld positionally at high speed and to make small fillet welds with a mitred profile that would be difficult to achieve by any other means. Despite this, efforts to sell such wires in Europe have generally been unsuccessful.
It is relatively easy to make self-shielded stainless flux-cored wires because their high chromium content increases the solubility of nitrogen in the weld metal and reduces the risk of nitrogen-induced porosity. However, the inferior handling characteristics of such wires compared to the gas-shielded types has led to a gradual decline in their popularity and they are now mainly used in the hardfacing, repair and maintenance sectors.
2.4.6 Wires for submerged arc welding
Although tubular wires for submerged arc welding have been on the market for more than 30 years, and the benefits claimed for them today were being discussed from the outset, they made very little impact until the 1990s. As with gas-shielded tubular wires, the deposition rate in submerged arc welding increases when a tubular wire is substituted for a solid wire at the same welding parameters, typically by 20-25 %. In multipass welds, this benefit comes with no penalty other than the extra cost of the wire itself.
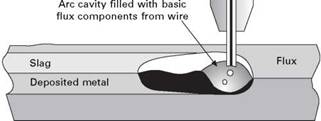
An interesting option, discussed for 30 years but only now achieving commercial success, is the possibility of using relatively acid submerged arc flux in combination with a basic tubular wire. Acid fluxes give a good bead appearance and slag detachability, and are less susceptible to moisture pickup than basic fluxes, but do not generally produce the tough welds needed, for example, in offshore applications. However, if used with a basic flux-cored wire, all the good attributes of the flux are retained, while the small amount of basic components, delivered directly to the arc cavity, lower the weld oxygen level and allow good toughness to be achieved. The process is shown in Fig. 2.2. The combination of acid and fused fluxes with basic tubular wires has now been taken up by the offshore industry and has many potential applications in other sectors.
|
2.2 Using a basic tubular wire with an acid flux in submerged arc welding. |
Users of tubular wires will not always need to know the details of how they are manufactured, but a brief explanation may help to show why wires
|
|
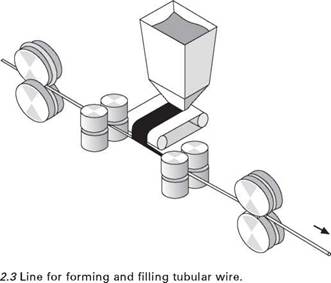
sometimes look and behave differently. Most wires are made by starting with a flat steel strip and rolling it to form a tube. At the point where it is U - shaped, powder is poured into it (Fig. 2.3). After the tube has been closed, which is typically at a diameter of a few millimetres, there are different ways of reducing the wire to its final diameter. It may be drawn through dies, like solid wires. In that case, the dies must be lubricated and special drawing soaps are available for the purpose. However, these compounds contain hydrogen and to prevent this finding its way into the weld, the wires have to be baked to remove the organic components of the soap by oxidation, ideally leaving a non-hygroscopic residue. By careful selection of the soap, this residue can also be made to act as a cathode stabiliser, allowing the wire to operate with electrode negative when needed.
Alternatively, the wire diameter may be reduced by rolling. This requires much less lubrication, so no baking is needed after production. This results in a cleaner wire surface which gives a lower electrical resistance at the contact tip, so the voltage delivered to the wire is more constant and the amount of arc stabilisers in the wire can be reduced, further lowering its potential hydrogen content.
It is possible to make seamless tubular wires by starting with a tube of 12mm or so in diameter and 15m in length, and filling it from one end. A series of filled tubes are then joined end-to-end before being drawn down to the final diameter. Because this typically involves a 10:1 reduction of diameter, an intermediate heat treatment at a high temperature is needed and this restricts the use of reactive materials, for example some deoxidants, in the core, which the developer of seamed wires might use. However, seamless wires can be coppered for lower contact resistance and should not pick up moisture during storage.