Solar Hydrogen Production From Aqueous Solutions. Of Ethanol At Near Ambient Temperatures
C. M. Rangel1*, R. A. Silva1, T. I. Paiva1 and V. R. Fernandes1
1 INETI, Department of Materials, Campus do Lumiar do INETI, 1649-038 Lisbon, Portugal
* Corresponding Author, :armen. rangel@.ineti. pt
Abstract
Nanostructured semi-conductor materials based on titanium dioxide, with effective photo-catalytic properties under UV illumination, were synthesized and characterized with the objective of studying the photo-catalytic hydrogen production from water. The need to decrease the electron - hole recombination rate was accounted for by metal doping. Ethanol was used as a hole trap. Aqueous suspensions of the semiconductor powders, with noble metal loadings (Pt) were used for a selected catalyst concentration. Hydrogen production was found to be linear with UV irradiation time at near room temperature. pH variations during hydrogen production were followed and associated to the formation of acetic acid during the reaction.
Keywords: hydrogen production, doped-titanium dioxide, ethanol, photo-catalytic materials.
1. Introduction
The generation of hydrogen from water splitting using photo-catalytic surfaces of oxide materials has been recognized since the early seventies [1]. In the last decades interest in semiconductor photocatalysis has grown significantly, with works mostly referring to uses in water/air purification.
The photo-catalytic production of hydrogen by means of irradiation of a suspension of semiconductor oxides, presents attractive features over other methods with higher cost such as water electrolysis.
Some of the materials properties and requirements for solar hydrogen production include tailored electronic structure: band gap - essential for absorption of solar energy; and flat band potential - must be higher than the redox potential of the couple H+/ H2. Furthermore, efficient charge transport is necessary since low electrical resistance is required as well as effective charge separation and prevention of electron-hole pair recombination.
Titania is the base catalyst material of choice, notwithstanding the stability and non-corrosive properties, and the environment friendliness and low cost, the actual efficiency in the production of solar hydrogen is still very low, due to electron-hole pair recombination [2-6] and also due to TiO2 band gap (~3.2 eV) which only allows utilization of UV light.
The feasibility of photo electrochemical generation of solar hydrogen requires that the energy
conversion efficiency goes from current levels < 1% to levels of > 10%, with accompanying
durability. In order to increase efficiency in the use of semiconductor electrodes in electrochemical photolysis, integrated systems including semi-conductor / redox couples interfaces, deposition of metallic co-catalyst, sensitizers, etc. have been studied [2,3]. The modification of TiO2 properties may contribute for a more efficient hydrogen production that may take advantage of visible light utilization [4,7,8,9]. Effective charge transfer from water molecules and the TiO2 lattice requires the presence of surface active sites, associated to point defects, that can form activated complexes with water molecules.
In this work, a nanostructured semi-conductor material based on titanium dioxide, with effective photo-catalytic properties under UV illumination, was used for hydrogen production using ethanol as a sacrificial agent, with excellent results.
A photochemical reactor with a total volume of 4.40 litters distributed between an internal (irradiated) reactor and an external reactor (fluid reservoir) was used according to need; a sensing pH electrode was allowed for as well as facilities for titration of H+, in order to adjust pH, when required. The internal reactor was contained in a black box and used a 450 W Hg immersion lamp (A. C.E. Glass Incorporated, NJ), as a radiation source. The emission spectrum of the lamp indicated that the UV radiation is mainly situated between 313 and 366 nm. Circulation between reactors, when required, was ensured by a peristaltic pump, according to the required recirculation rate. Agitation by magnetic stirrers in both reactors was also used.
In this paper, titanium dioxide Degussa P-25 was modified by photochemical deposition of Pt. Platinised TiO2 catalyst (at 1.5 wt.% Pt) was prepared using hexachloroplatinic acid (Riedel-de Haen) as the precursor. A pre-determined amount of TiO2 was first suspended in hot water and the hexachloroplatinic acid previously dissolved in an aliquot of fresh distilled water was added, with continuous nitrogen purging (15 min.) inside the described photo-reactor. The mixture was irradiated for 60 min, at constant temperature (30-40°C), to ensure that all the platinum in the suspension was reduced and deposited onto the surface of TiO2. The TiO2 / Pt catalyst was subsequently recovered by filtration and washed repeatedly with water. Finally, the powder was dried at 70°C and stored under vacuum in a desiccator. Catalyst were heat treated at 440 °C during one hour.
After some preliminary studies a concentration of TiO2 of 0.5 gL-1 was selected as well as a concentration of ethanol of 5 M and an initial pH of 11[10].
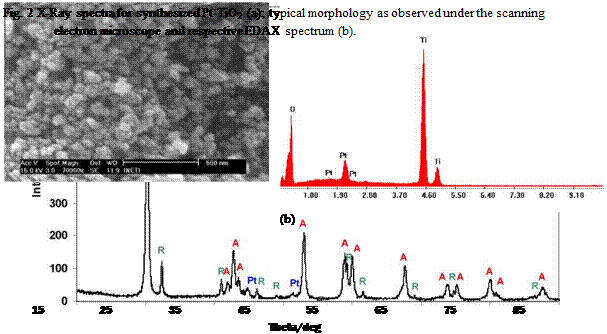
Characterisation of the powders was done by X-Ray diffraction using a Rigaku, model D-Max IIIC and by scanning electron microscopy (SEM) using a Phillips model XL 30 FEG microscope coupled to EDS.
In this work, a nanostructured semi-conductor material with effective photo-catalytic properties under UV illumination was synthesized using a photochemical method, taking as a base Degussa P-25 powder and platinum salts.
X-Ray diffraction data of the synthesized powder indicated the presence of anatase and rutile, see figure 2a). Anatase content was estimated to be ~ 89.21% and the crystallite size 21 nm. Typical morphology of the powder is shown in figure 2b), exhibiting particle size at a nanoscale.
Aqueous suspensions of the semiconductor powders, with noble metal loadings (Pt) of 1.5 wt% were used and the hydrogen production studied, for a fixed amount of the catalyst of 0.5 g/L. A 5M concentration of ethanol was used.
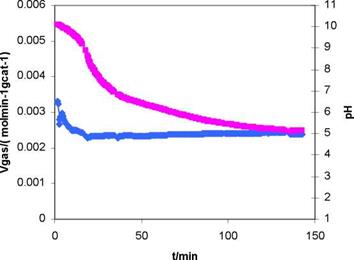
Typical results obtained during the photocatalytic production of hydrogen by UV illumination of the suspension of TiO2-Pt are shown in figure 3. The rate is presented in terms of the number of mol per unit time of produced gases by gram of catalyst.
A titration confirmed the presence of large amounts of acetic acid, indicating that pH variations during the reaction must be significant. Typical pH variations with irradiation time were measured starting from the initial pH value of 11 falling to 5.1, see figure 3.
|
Fig. 3 Gas production rate as a function of time, from a 5 M aqueous solution of ethanol at pH 11, using own synthesized Pt-TiO2 . The pH solution variation is also shown. |
|
Possible reactions are: |
|
|
hv |
|
|
C2H5OH + H2O ^ CH4 + CO2 + 2H2 |
(1) |
|
ТІО2 |
|
|
e (Me) + H+ sol ^ Hads |
(2) |
|
H2ads ^ H2 gas |
(3) |
|
2h + C2H5OH ^ 2H+ + CH3CHO |
(4) |
|
2h + CH3CHO + H2O ^2 H+ + CH3COOH |
(5) |
Where Me - Metal (Pt); h - hole
Methane and CO2 can be produced by the decomposition of acetic acid, h + CHaCOO" ^ CO2 + CH30 (6)
Another pathway for CH4 formation may be the hydrogenation of CO2 according to equation (7)
![]() CO2 + 4 H2 ^ CH4 + H2O
CO2 + 4 H2 ^ CH4 + H2O
Another possible reaction pathway is the involvement of adsorbed surface hydroxyls on TiO2 in the trapping of holes. The interaction of surface hydroxyl groups with holes will result in the formation of hydroxyl radicals which, in turn, will interact with C2H5OH or its intermediates adsorbed on the metal - TiO2 surface or present in the vicinity to produce CO2 and other side products.
Another possible reaction pathway is the involvement of adsorbed surface hydroxyls on TiO2 in the trapping of holes. The interaction of surface hydroxyl groups with holes will result in the formation of hydroxyl radicals which, in turn, will interact with C2H5OH or its intermediates adsorbed on the metal - TiO2 surface or present in the vicinity to produce CO2 and other side products.
Work proceeds with analysis of the gas mixtures by gas chromatography.
Work in progress includes comparison with own synthesized sol-gel titanium dioxide modified with Pt in the similar experimental conditions. Modification of the titanium dioxide band gap is also in progress, in order to account for the advantageous use of visible light [11].
Titania was used as the base catalyst material of choice for solar hydrogen production, due to its stability and non-corrosive properties, as well as environmentally friendliness and low cost. The need to decrease the electron-hole recombination rate was accounted for by platinum deposition and the addition of ethanol as a hole trap.
o Gas production rates were found to be linear with time at near room temperature.
o pH variations during hydrogen production were striking changing from 11 to values as low as 5.6, this is thought to be due to the formation of acetic acid during the reaction, accounting for the lower concentration of CO2 and CH4 found by gas chromatography Keeping the pH in the alkaline range ensured a constant rate of gas production for extended periods of time.
Further work is needed to identify low cost metal loading materials with acceptable enhancement for hydrogen production, as well as modified catalysts that allow effective utilization of visible light.
Acknowlegments
Acknowlegments are due to B. Charasse, J. Chesnau and M. Pinho for assistance in some of the experiments.
References
[1] A. Fujishima, A Honda, Nature, 238 (1972), 37-38.
[2] - Y. Z. Yang, C. H. Chang, H. Idriss, Appl. Catal. B, 67 (2006) 217-222.
[3] - G. R. Bamwenda, S. Tsubota, T. Nakamura, M. Haruta, J. Photochem. Photobiol. A, 89 (1995) 177-189.
[4] - A. R. Gandhe, J. B. Fernandes, Bull. Catalysis Society of India, 4 (2005) 131-134.
[5] - S. Pilkenton, Son-Jong Hwang, D. Raftery, J. Phys. Chem. B, 103 (1999) 11152-11160.
[6] - Z. Zaina, L. K. Hui, M. Z. Hussein, Y. H. Taufiq-Yap, A. H. Abdullah, I. Ramli, J. Hazardous Materials, B125 (2005) 113-120.
[7] - J. C. Kennedy III, A. K. Daty, J. Catalysis, 179 (1998) 357-389.
[8] - G. Marci, V. Augugliaro, A. B. Prevot, C. Baiocchi, E. Garcia-Lopez, V. Loddo, L. Palmisano, E. Paramauro, M. Schiavello, Societa Chimica Italiana, Annalli di Chimica, 93 (2003) 693-644.
[9] - C. G. Silva, W. Wang, J. L. Faria, J. Photochem. Photobiol. A, 181 (2006) 314-324.
[10] - R. Gouveia, R. A. Silva, C M. Rangel, Mat. Sci. Forum, 514-516 (2006) 1385-1390.
[11] - Y Li, C. Xie, D. Peng, G. Lu, S. Li, J. Mol. Catal.: A Chem., 282 (2008)117-123.