Solar energy in progress and future research trends
Conduction
It is the heat transfer within a solid body where there are at least two different heat areas, i. e. temperature difference. Such a heat transfer is possible by means of vibrations of the atomic lattice, which forms the body of the material. The heat is also carried away by electrons, and this contribution is much greater than that due to lattice vibration. During conduction there is no mass transfer. Atoms move randomly under thermal stress in liquids and gases, and they also lead to heat conduction. The heat transfer is proportional with the temperature difference along a distance (temperature gradient) and hence the heat flow by conduction can be expressed by the following mathematical equation
Hf = — k(dT/dx) (68)
where Hf is the heat flow per unit area of cross section (W/m2), T is the temperature (°C), x is the direction and distance (m), and k is the thermal conductivity of the material (W/m °C). Thermal conductivity is special for
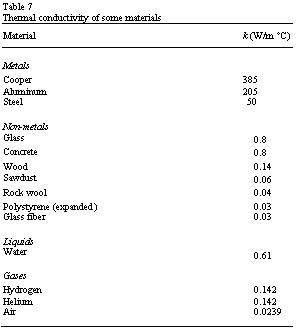
 each material and its value is given for various materials in Table 7.
each material and its value is given for various materials in Table 7.
As solar radiation absorbed by opaque materials, the energy redistributes itself because it is conducted between adjacent molecules. Such redistribution is dependent on temperature difference and the thermal conductivity of the material. Metals, in general, have big conductivities and consequently can transmit large amounts of energy under small temperature differences (temperature gradients). In insulators the reverse situation is valid where under large temperature gradients only a small amount of heat is conducted. It is known that the air is a very good insulator. Hence, most of the practical insulators rely on very small pockets of air traps between the panels of glazing as bubbles in a plastic medium or between the fibers of mineral wools.
12.2. Convection
This is a process by which heat from the hot surfaces is carried away by a fluid such as water flowing fluid across the surface is heated and then the heated volume is removed due to fluid flow with replacements of new and cold fluid. This heat transfer is referred to as convective cooling or heating. The rate of heat removal depends on both the temperature difference between the surface and the bulk fluid temperature and also on the velocity and characteristics of the fluid. Another sort of convective heat transfer can be considered for a horizontal hot plate in still air, where the air adjacent to the top surface will become hotter than the bulk of air temperature. As a result of hot air expansion and density decrease, hot air is replaced by cooler air. In solar energy conversion both forced and natural convections may be
accompanied by phase changes. Hence, the convective heat transfer can be expressed by the following relation
Hf = h(Ts - Tf) = h DT (69)
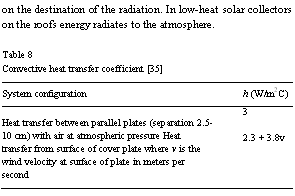
where Hf is the heat flow per unit area (W/m2), h is the convective heat transfer coefficient (W/m2 C), Ts is the surface temperature (8C), Tf is the fluid temperature (8C), and DT = Ts — Tf. The actual calculation of h is somewhat complicated, because it is dependent on both the nature of the fluid and also on its flow velocity. Approximate convective heat transfer coefficients are given for flat plate collector in Table 8.
This refers to the transference of heat to a fluid (gas or liquid). Energy is transferred to molecules of the fluid, which then physically move away taking the energy with them. A warmed fluid expands and rises creating a fluid known as natural convection, which is one of the principle processes of heat transfer through windows. It occurs between the air and glass. It is possible to reduce the convection losses through double-glazing windows by filling space between the double-glazing with heavier, less mobile gas molecules, such as argon or carbon dioxide. On the other hand, since the convection currents cannot flow in a vacuum, the space between the double-glazing may be evacuated.